Abstract
Background Chimeric antigen receptor (CAR-) T cell therapy showed enormously promising results in patients with relapsed/refractory multiple myeloma (rrMM), leading to approval of the first 2 products by FDA/EMA. Patients with rrMM who receive CAR-T cell therapy may have received several lines of prior therapy and experienced multiple relapses. Therefore, patient selection is especially important for CAR-T cell therapy, a very complex and expensive treatment, which recently showed risks for treatment delay or even cancellation due to long turnaround times of apheresis and infusion. Here, we summarized the current body of evidence on the role of CAR-T cell therapy for rrMM and high-risk disease, to identify patients who may benefit and those who may not.
Methods We performed a systematic literature review to identify all fully published prospective trials. We searched Medline, EMBASE, Cochrane trials registry, and www.clinicaltrials.gov. A conventional meta-analysis was conducted using R statistical software. Risk ratios (RR) and 95% confidence intervals (CI) were calculated within a random-effects framework. Main efficacy outcomes were overall response (ORR), complete response, minimal residual disease, survival, and relapse. High-risk disease features were defined as presence of the following at time of CAR-T cell infusion: cytogenetic high-risk (presence of at least either del(17p) or t(4;14)), presence of extramedullary disease (EMD), Revised International Staging System (R-ISS) stage III.
Results 19 trials comprising a total of 759 patients with heavily pretreated rrMM were included in quantitative analyses. Patients received a median of seven prior treatment lines (such as proteasome inhibitors, immunomodulatory drugs, monoclonal antibodies), including autologous stem cell transplantation in 80% of patients. The median age of patients was 60 years. Age and number of prior lines of therapy was significantly affected by trial origin. Median age and lines of therapy were 61 and 7 lines in US-based trials versus 57 years and 5 lines in China-based trials.
The most frequent single target was B cell maturation antigen (BCMA). 4 trials used tandem CARs, including 2 trials targeting both BCMA and CD38 and 2 trials targeting both BCMA and CD19. 1 trial only targeted CD19. Costimulatory domain was 4-1BB designs in most trials, and fludarabine and cyclophosphamide was the most common lymphodepletion regimen.
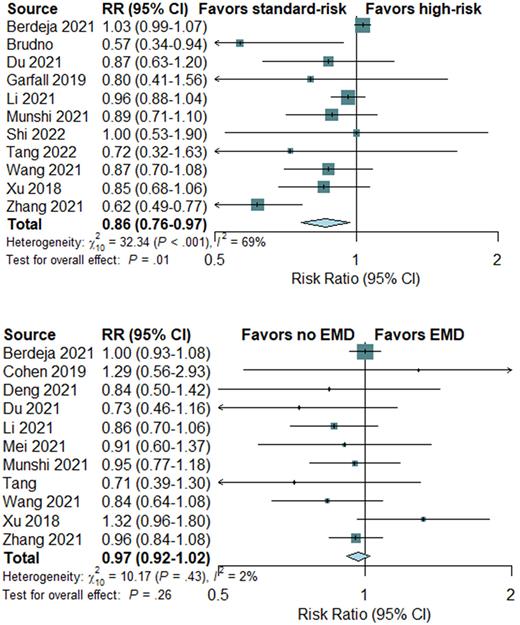
In terms of response, high-risk cytogenetic profile was significantly associated with worse ORR, showing RR of 0.86 (95% CI, 0.76-0.97) in favor of standard-risk cytogenetics (P=0.01; Figure). Results for complete response and measurable residual disease were in line with these results and all in favor of standard-risk cytogenetics, showing 30% and 40% increased risk for worse outcome (P<0.001, respectively). Regarding disease risk, EMD was not significantly associated with worse ORR, showing RR of 0.97 (95% CI, 0.92-1.02; P=0.26; Figure). No significant associated with complete response and measurable residual disease were seen. R-ISS stage III was significantly associated with worse ORR, complete response, and measurable residual disease (P<0.001, respectively).
In terms of relapse, high-risk cytogenetics and EMD were significantly associated with worse outcome, showing RRs of 0.70 (95% CI, 0.58-0.83; P<0.001) and 0.88 (95% CI, 0.78-0.98; P=0.02). This translated into significant associations with worse progression-free survival. Overall survival could not be sufficiently assessed due to missing reports, although 2 trials suggested worse outcomes at least for EMD.
Conclusion High-risk cytogenetics were significantly associated with worse outcomes after CAR-T cell therapy for rrMM. Although EMD was not associated with significantly different ORR, risk for relapse and worse survival was significantly increased.
Disclosures
Ayuk:Takeda: Honoraria; Mallinckrodt/Therakos: Honoraria, Research Funding; Miltenyi Biomedicine: Honoraria; Novartis: Honoraria; Janssen: Honoraria; Medac: Honoraria; Gilead: Honoraria; Celgene/BMS: Honoraria. Kroeger:Novartis: Honoraria; Kite/Gilead: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal